Background: Patients with Core-Binding Factor (CBF) and NPM1-mutated acute myeloid leukemia (AML) are considered as favorable or intermediate-risk according to the European LeukemiaNet (ELN) 2022 classification and allogeneic hematopoietic cell transplantation (HCT) is usually not considered in those achieving first complete remission (CR) with good molecular response. Close molecular monitoring by quantitative PCR has been widely implemented over time and approximately 30% of patients will experience molecular relapse. However, there is no recommendation on how to manage these patients due to the lack of data from large patient series or clinical trials. In this study, we retrospectively analyzed the outcome of CBF or NPM1-mutated AML patients who had MRD monitoring after having achieved first CR.
Methods: All CBF and NPM1-mutated AML patients, aged 18-60 years, who had MRD molecular monitoring (RT-qPCR of RUNX1-RUNXT1 or CBFB-MYH1 transcripts or classical NPM1 mutations) after first-line therapy including induction therapy with daunorubicin/idarubicin and cytarabine followed by intermediate or high-dose cytarabine consolidation, without allogeneic HCT in first CR, were included. MRD measurements were generally performed every three months during follow-up for 24 months in either bone marrow (BM) or peripheral blood (PB). Molecular relapse was defined according to ELN recommendations (Heuser M, Blood 2021) as a conversion from MRD negativity to positivity or an increase in MRD copy numbers >1 log between two positive samples.
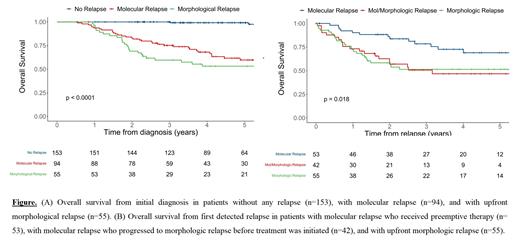
Results: Between 2010 and 2019, 303 patients from 9 centers with newly diagnosed CBF or NPM1-mutated AML ( RUNX1-RUNX1T1, 19%; CBFB-MYH11, 27%; NPM1, 53%) and available MRD monitoring after first-line therapy were included. Among these patients, 153 (51%) never relapsed, 95 (31%) had molecular relapse, with a median of 326 days (interquartile range [IQR]: 276-438) from initial diagnosis, and 55 (18%) had upfront morphologic relapse (i.e., without prior molecular relapse detected), with a median of 388 days (IQR: 280-559). Patients with molecular and upfront morphologic relapse had higher white blood cell (WBC) count at diagnosis (12 vs. 22 vs. 30 G/l in those without relapse, molecular relapse, and morphological relapse, respectively, P=0.003) and were more likely to have a FLT3-ITD mutation (7% vs. 25% vs. 19%, respectively, P=0.001). In addition, patients with upfront morphologic relapse had a lower log-reduction of MRD during first-line treatment (4.90 vs. 4.93 vs. 3.77, respectively, P=0.009) and were more likely to have CR with positive MRD at the end of treatment in peripheral blood (2% vs. 16% vs. 32%, respectively, P<0.001). Overall survival (OS) was significantly different according to whether patients never relapsed, had molecular relapse, or had upfront morphological relapse (three-year OS of 100% vs. 75% vs. 60%, P<0.001; Figure A). Among the 95 patients with molecular relapse, 53 (56%) received preemptive therapy (median of 48 days [IQR:24-68] from molecular relapse to treatment initiation) and 42 (44%) progressed to morphologic relapse before treatment was initiated (median of 58 days [IQR:36-134] from molecular to morphologic relapse). Patients who received preemptive therapy had a better OS than those who received salvage therapy after having progressed from molecular to morphologic relapse, and those who had upfront morphologic relapse (78% vs. 51% vs. 51% at three years, respectively, P=0.01; Figure B). Preemptive therapy included upfront allogeneic HCT (n=19), intensive chemotherapy (n=21), and non-intensive therapy (n=13) with different OS rates that did not reach statistical significance (92% vs. 79% vs. 58% at three years for upfront allogeneic HCT, intensive chemotherapy, and less intensive therapy, respectively, P=0.09).
Conclusion: Up to 30% of patients with CBF or NPM1-mutated AML experienced molecular relapse in our cohort and more than 50% of them received preemptive therapy. Patients treated preemptively at molecular relapse had a better OS than those treated with active disease. Although these results need to be confirmed in larger studies, it supports molecular monitoring during follow-up to start preemptive therapy before overt morphologic relapse. Other techniques for MRD monitoring are urgently needed for AML patients who cannot be monitored by qPCR.
Disclosures
Bertoli:Abbvie: Honoraria, Other: Travel; Astellas: Honoraria; BMS-Celgene: Honoraria; Jazz Pharmaceuticals: Honoraria, Other: Travel; Servier: Honoraria; Novartis: Honoraria. Dumas:Astellas: Honoraria, Other: Research support for institution; Abbvie: Honoraria; BMS: Honoraria, Other: Research support for institution; Servier: Honoraria, Other: Research support for institution; Novartis: Honoraria, Other: Research support for institution; Daiichi-Sankyo: Honoraria, Other: Research support for institution; Jazz pharmaceutical: Honoraria; Janssen: Honoraria; Roche: Other: Research support for institution. Pigneux:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings; Gilead: Honoraria; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees. Mathilde:Novartis: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Clinigen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Other: Support for attending meetings.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal